How amorphous glass can form
complex shapes
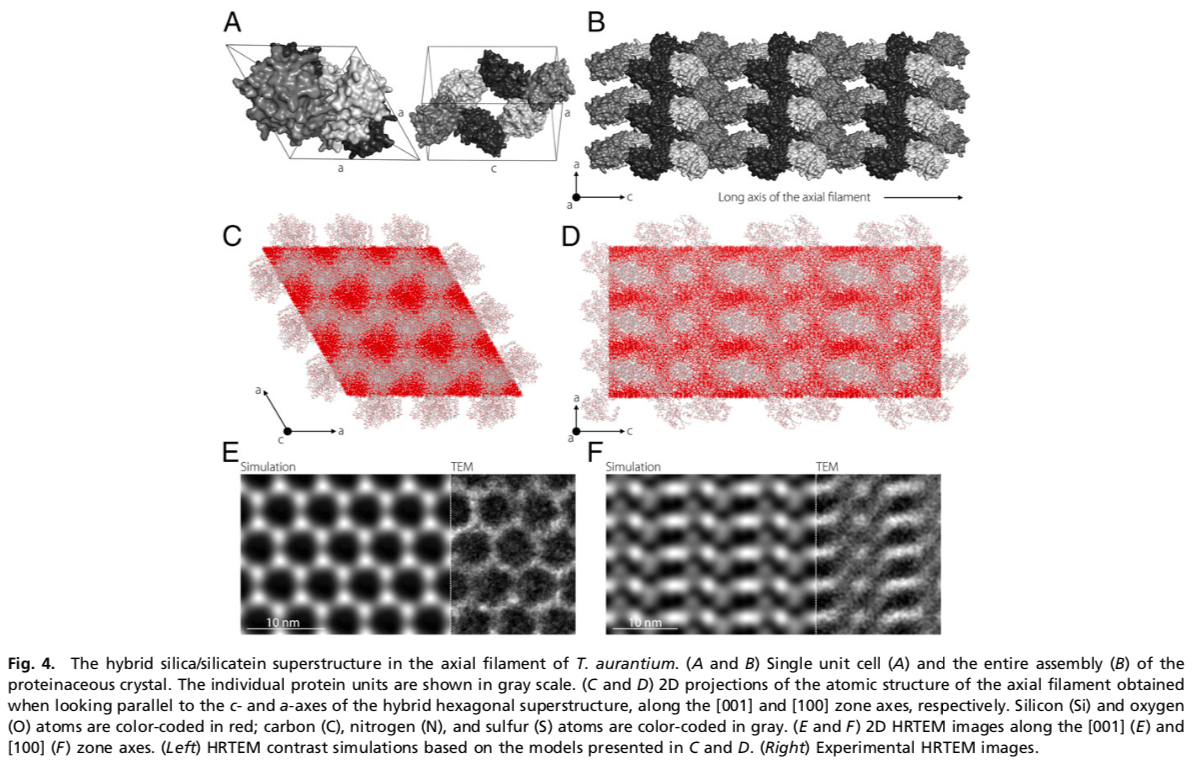
Formation of highly symmetric skeletal elements in demosponges, called spicules, follows a unique biomineralization mechanism in which polycondensation of amorphous silica is guided by a proteinaceous scaffold, the axial filament. The enzymatically active proteins, silicateins, are assembled into a slender hybrid silica/protein crystalline super- structure that directs the morphogenesis of the spicules. Despite the biological and biotechnological importance of silicatein, its tertiary structure was never determined. In our article published in PNAS, we report the atomic structure of silicatein and the entire mineral/organic hybrid assembly with a resolution of 2.4 Å. In this work, the serial X-ray crystallography method was successfully adopted to probe the 2-μm-thick filaments in situ, being embedded inside the skeletal elements. In combination with imaging and chemical analysis using high-resolution transmission electron microscopy, we provide detailed information on the enzymatic activity of silicatein, its crystallization, and the emergence of a functional three-dimensional silica/protein superstructure in vivo. Ultimately, we describe a naturally occurring mineral/protein crystalline assembly at atomic resolution. (Görlich et al. 2020. Natural hybrid silica/protein superstructure at atomic resolution. PNAS Nov 2020, 202019140; DOI: 10.1073/pnas.2019140117 ).